You probably haven’t heard of “multiferroics,” but they could lead to entirely new ways of designing technologies, some of which we are only just starting to imagine
To most of us, the word “civilization” is synonymous with enlightenment, culture or refinement; it conjures up images of grand public buildings or brings to mind advanced social systems. But to my mind, civilization is nothing more than a measure of the state of progress in materials science, because from the Stone Age through the Bronze to the Iron Age, every major advance in human civilization has been driven by a fundamental development in materials. The association is so strong that we even name our historical eras after the materials that dominated at the time.
In today’s “Silicon Age,” silicon transistors form the core of much of the microelectronics that enable our modern way of life. And over the past few decades, we have improved the properties of silicon devices to an astonishing extent, enabling the transformation of clunky old desktop computers into sleek smartphones while taking for granted the exponential increase in capability—and corresponding decrease in size and cost—as captured by Moore’s Law.
But this silicon revolution will soon be forced to come to an end as we start to run into fundamental physical limits set by the size of the individual atoms that make up the silicon material. And this means that the steady march toward faster, smaller, lighter products with more and more functionality can’t continue within our existing framework.
Although it might seem that our electronic devices are already light and small and powerful enough, this roadblock is in fact a profound problem for society. Worldwide use of microelectronics is expanding so rapidly that, by many projections, more than half of the world’s energy will be consumed by information technologies within a couple of decades. And this consumption is not sustainable. To maintain and improve our global standard of living, we need to step beyond the Silicon Age, and to do this we need a new material.
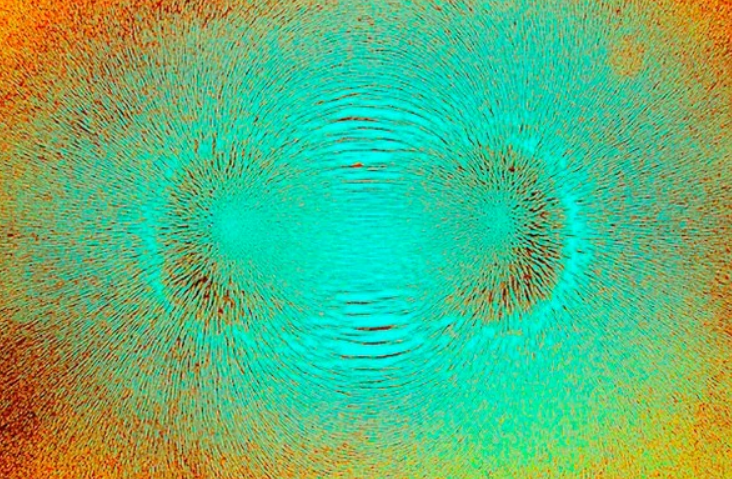
Twenty years ago, I was a young postdoctoral researcher working on ferromagnetic materials—these are materials that contain magnetic dipoles with their north and south poles lined up in the same direction—in a research group that specialized in ferroelectric materials, which are materials with aligned electric dipoles made of positive and negative charges. My plan was to take the techniques that my host group had developed to study ferroelectric materials and apply them to the study of ferromagnets; the “ferro” in both names reflects similarities in the underlying physics between the two material classes.
I noticed, though, that the kinds of materials I was working on were different from those of my colleagues. For example, most ferromagnetic materials are black metals, like iron, whereas most ferroelectric materials are transparent ceramics. The question “Why are there so few magnetic ferroelectrics?” intrigued me to the extent that finding its answer became the focal point of my research program.
What I discovered was very simple: the atoms that form the kinds of chemical bonds needed to produce magnetic dipoles in a material have different chemistries from those that tend to make electric dipoles. But there is no fundamental reason why the two phenomena can’t be combined. And armed with this understanding, my collaborators and I were able to develop a new class of materials called multiferroics, which indeed are magnetic and ferroelectric.
So why are multiferroics important? Well, magnetic materials are complementary to silicon in today’s technologies—where silicon is used for processing information, magnetic materials are used for storing it, by representing the 1s and 0s of digital electronics through opposite orientations of their magnetic dipoles. And magnetic materials are very good at this, because these magnetic data bits are stable, small and can be accessed quickly. Yet magnetic devices come with a cost—producing the magnetic fields that are needed to control the magnetism requires bulky components and uses a lot of energy.
But imagine the possibilities offered by a material that is both magnetic and ferroelectric. Multiferroic materials still have all of the advantages of magnetic materials, but in addition their magnetism can be controlled using electric fields. And compared with magnetic fields, electric fields are efficient: they can be made of tiny components, and they use vanishingly small amounts of energy. Our new multiferroic materials are poised to enable entirely new device paradigms, different ways of designing technologies along lines we are only just starting to imagine.
What are the walls that we need to break down so we can enter a new “Multiferroics Age”? We need to develop new materials in which the magnetic and electric dipoles remain stable at room temperature, even when we make them very small. We need to understand the process of reorienting the dipoles well enough to do it with very small electric fields. And we need to make sure that our new materials are abundant on the earth, environmentally benign, and cheap and easy to process. And in return, our multiferroics are providing us with a playground for exploring a host of exciting fundamental scientific questions, as well as maybe, just maybe, paving the way to the next materials age.
Editor’s note: This post was produced in partnership with the annual Falling Walls Conference in Berlin, which coincides with the anniversary of the fall of the Berlin Wall and showcases the work of researchers from around the world, including the author of this essay.










RSS